Good Manufacturing Practice GMP
What is GMP?
Good Manufacturing Practice (GMP) is a term that is recognized worldwide for the control and management of manufacturing and quality control testing of foods, pharmaceutical products, and medical devices.
Why GMP?
GMP requirements concern methods, equipment, or testing, which are used for the production, processing, packaging, and/or storage of products. This ensures that regulated products fulfill the necessary quality criteria. At the same time, the GMP regulations have an increasing influence on suppliers of raw materials, packaging materials, manufacturing facilities, and testing equipment. The compliance of GMP regulations is constantly examined by inspectors of health care system authorities.
These regulations, which have the force of law, require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective. GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-ups, and errors. This, in turn, protects the consumer from purchasing a product that is not effective or even dangerous. Failure of firms to comply with GMP regulations can result in very serious consequences including recall, seizure, fines, and jail time.
CGMP :
GMP is also sometimes referred to as “CGMP”. The “c” stands for “current,” reminding manufacturers that they must employ technologies and systems which are up-to-date in order to comply with the regulation. Systems and equipment used to prevent contamination, mix-ups, and errors, which may have been “top-of-the-line” 20 years ago, maybe less than adequate by today’s standards.
Thus, Good Manufacturing Practices –GMP, when certified of an organization, is an authorization and certification of Companies product & process that quality standards are adequate, up to date, and controlled for the intended use by the consumer.
Steps for GMP Implementation and Certification
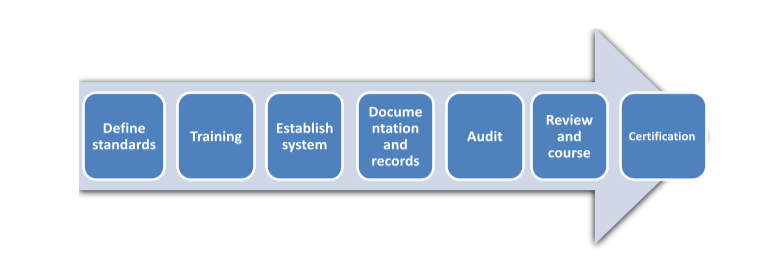
GMP Consulting Service:
AISC provides Good Manufacturing Practices GMP consulting services to industries. We can provide and help you to implement or comply with GMP through;
- GAP assessment.
- GMP Validation Protocols
- SOP & Documentation.
- GMP training.
- Change Control program.
- Audit.
Benefits of GMP
- Final testing of the product cannot ensure Quality efficiency and safety.
- Final testing may always not detect contamination, error, etc.
- Conformance to the predetermined specification.
- To minimize contamination eg:- microbial contamination.
- To eliminate error.
- To produce products of consistent quality.
- Government requirement.
- Ensure quality product.
- Reduce rejects, recalls.
- Satisfied customers.
- Company image and reputation.
Main Risks Without Gmp:
- Unexpected contamination of products, causing damage to health or even death.
- Incorrect labels on containers could mean that patients receive the wrong medicine.
- Insufficient or too much active ingredient, resulting in ineffective treatment or adverse effects.
Principles Of Gmp:
- Design and construct the facilities and equipment properly
- Follow written procedures and Instructions
- Document work
- Validate work
- Monitor facilities and equipment
- Write step by step operating procedures and work on instructions
- Design, develop and demonstrate job competence
- Protect against contamination
- Control components and product-related processes
- Conduct planned and periodic audits

